Chennai man developed neurological issues after getting COVID-19 vaccine: Doctor


Send us your feedback to audioarticles@vaarta.com


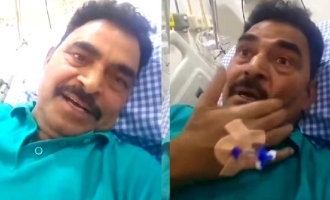
A Chennai-based neurologist from Apollo Hospitals has noted that a 40-year-old businessman in the city has developed acute neurological symptoms after receiving the Oxford Covishield Covid-19 vaccine during the third phase of the vaccine trial conducted by the Serum Institute of India (SII), Pune on November 11.
The 40-year-old man visited the doctor for consultation after he was discharged from Sri Ramachandra Medical college and research institute (SRMC) in Chennai on October 1, where he was administered a shot of Covishield. "The conglomerates of clinical, electro physiological and neuro psychological examinations, in the absence of any other diagnosable modalities, the neurological dysfunction suffered by the patient subsequent to his vaccination relates to immunogenicity of COVISHIELD-Covid19 vaccine," the doctor noted on November 21. A copy of the declaration was also reportedly cited in the volunteer's legal notice to SII.
The doctor had decided that the vaccine was responsible for the patient's neurological damage after he apparently eliminated every other possibility that could result in the condition through a series of tests, including electroencephalogram (EEG), somato sensory evoked potential (SSEP) test and neuropsychological assessment. Following the doctor's assessment, a law firm representing the 40-year-old participant sent a legal notice to SII seeking a compensation of Rs. 5 crore, in addition to demanding that manufacturing and distribution of the Covid-19 vaccine be stopped.
Responding to the volunteer's claims, SII stated, "The COVISHIELD vaccine is safe and immunogenic. The incident with the Chennai volunteer though highly unfortunate was in no way induced by the vaccine and Serum Institute of India is sympathetic with the volunteer's medical condition. However, we would like to clarify that all the requisite regulatory and ethical processes and guidelines were followed diligently and strictly. The concerned authorities were informed and the Principal Investigator, DSMB and the Ethics Committee independently cleared and reckoned it as a non-related issue to the vaccine trial. Post which we submitted all the reports and data related to the incident to the DCGI."
The company has also filed a defamation lawsuit of Rs. 100 crore against the 40-year-old volunteer for slandering them. "It is only after we cleared all the required processes that we continued with the trials. We would want to assure everyone that the vaccine won't be released for mass use unless it is proven immunogenic, and safe. Taking into consideration the complexities and existing misnomers about vaccination and immunisation; the legal notice was sent therefore to safeguard the reputation of the company which is being unfairly maligned," the statement further read.
Follow us on Google News and stay updated with the latest!




 Follow
Follow